54+ calculate the percent of chlorine in barium chloride.
Web Convert mole of each element to mass of each element. In System International SI units the concentration of compounds is measured in moles and mole-derived units per liter and liter-derived units.

On Analysis A Certain Compound Was Found To Contain Iodine And Oxygen In The Ratio Of 254 80 The Formula Of The Compound Is Atomic Mass Of I 127 O 16
36 g H2O 2442 g Bac122H20 -.

. So 0129 x 6 0833 moles of oxygen. This calculator converts between units of mass and molar concentration for hundreds of. Web On an industrial scale barium chloride is prepared via a two step process from barite barium sulfate.
It can also be obtained from barium carbonate or barium hydroxide. 1 mol P 3097 g P 1 mol P 3097 g P 3 mol Cl 3545 g Cl 1 mol Cl 1064 g Cl Calculate the percent by mass of each element. Web Barium chloride dihydrate has a molar mass of 24426 g mol1 which means that one mole of hydrate has a mass of 24426 g.
The first step requires high temperatures. Web If the formula used in calculating molar mass is the molecular formula the formula weight computed is the molecular weight. Mass of sample 3097 g 1064 g 1374 g Percent P 3097 g 1374 g 100 2254 Percent Cl 1064 g 1374 g 100 7744.
National Library of Medicine. Since one mole of hydrate contains 2 moles of water and since water has a molar mass of 18015 g mol1 it follows that you get 2 18015 g of water for every 24426 g of hydrate. Web molar mass H 2O 2101 1600 1802 gmol.
When they react a barium atom will give up two electrons to form a action and a chlorine molecule will pick up two electrons to form a pair of chloride ions. From the chemical formula you will know different atoms and their number in a Barium chloride molecule. Web The overall chemical equation says that 1 mole of glucose reacts with 6 moles of oxygen gas for the reaction to occur.
The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom or group of atoms in the formula by the formula weight and multiplying by 100. From barium sulfate at high temperature BaSO4 4C BaS 4CO Step 2. However non-SI units are still used in many countries.
BaSO 4 4 C BaS 4 CO. Moles of Mg Cl O 910182728 moles. Assume the true value is the same as the theoretical value of percent water which can be calculated from the formula.
BaS 2 HCl BaCl 2 H 2 S. National Institutes of Health. Chemical formula of Barium chloride is BaCl 2.
C l X 2 2 e X 2 C l X. Web 54 calculate the percent of chlorine in barium chloride. Express the number of moles of Mg Cl and O atoms numerically separated by commas.
Percentage of water of hydration 2 1802 24434 100 1475. Web An experiment was done to calculate the percent of water in barium chloride dehydrate BaCl2 2H20. We can then proceed to calculating the percentage of water of hydration in barium chloride crystals.
A sample of propane C3H8 contains 127 moles of carbon atoms. Using these theoretical percentages calculate the theoretical yield of copper and chlorine that should have been produced based on the amount of compound. So the glucose to oxygen ratio is 16 or basically we need 6 times as many moles of oxygen gas as we do glucose for the reaction to happen.
Web The compound barium chloride is not the same thing as barium and chlorine mixed together. Convert to ppmv HCl ppmv 100 mgdscm x 0659 ppmv mgdscm 659 ppmvCl ppmv 10 mgdscm x 0339 ppmv mgdscm 34 ppmv 2 Step 2. Web calculate the percent chloride in an unknown chloride sampleMass sample 06794gMass AgCl and filter paper 24846gMass of filter paper 07798g This problem has been solved.
From the chemical formula of Barium chloride it is easy to find out that there are one. National Center for Biotechnology Information. From Barium sulphide BaS 2HCl BaCl2 H2S Chlorine can be used instead of hydrochloric acid.
B a B a X 2 2 e X. Web Calculate the number of moles of magnesium chlorine and oxygen atoms in 910 moles of magnesium perchlorate Mg ClO42. Molar mass BaCl2 2H 2O 1374 23545 22101 1600 24434 gmol.
The second step requires reaction between barium sulfide and hydrogen chloride. Find out the chemical formula and determine constituent atoms and their number in a Barium chloride molecule. Web Barium chloride BaCl2 CID 25204 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more.
Convert between mass and molar concentration. Web Using the formula of the compound and the atomic masses of copper and chlorine calculate the theoretical mass percent copper and the theoretical mass percent chlorine in the compound. Web Preparation of Barium Chloride Industrially it is obtained in two steps.
Web Answer 1 of 7. Or between barium sulfide and calcium chloride. When you have both of those things.
Barium Chloride At Best Price In Chennai By Mehta Chemical Industry Id. Convert to a Cl- equivalent Cl- Equivalent ppmv 659 ppmv 2 x 34 ppmv 73 ppmv Or alternatively using Equation 3. Youll get a detailed solution from a subject matter expert that helps you learn core concepts.
Jumat 17 Februari 2023 Web Calcium Chloride molecular weight Molar mass of CaCl2 110984 gmol Convert grams Calcium Chloride to moles or moles Calcium Chloride to grams Molecular weight. The results below are from the five trials. Web The percent composition of barium in barium chloride is 659 calculation percent composition molar weight of bariummolar weight of barium chloride x 100 from periodic table the molar weight of barium 1373 and the molar weight of BaCl2 1373 355 x 22083 composition is therefore 13732083 x 100.
Calculate to overall precision and accuracy from these data.
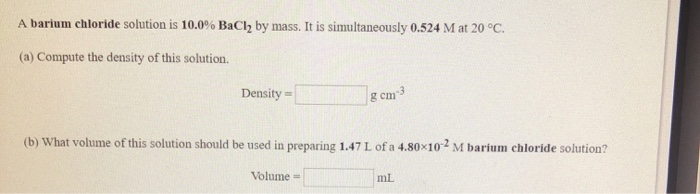
Solved A Barium Chloride Solution Is 10 0 Bacl2 By Mass It Chegg Com

Calculate The Percentage Of Chlorine In The Following Kclo3

Course Material Chemical Pdf Mill Grinding Sodium Carbonate

Pdf Natural Sciences In Archaeology And Cultural Heritage Book Compressed Ioannis Liritzis Academia Edu
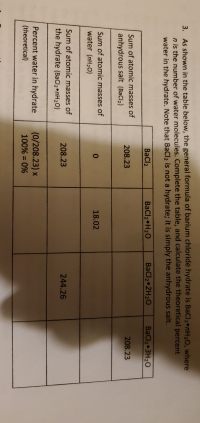
Answered 3 As Shown In The Table Below The Bartleby
Solved Solve This Chemistry Problem With Solution So That I Will Have A Course Hero
Barium Chloride Dihydrate

Batteries International Issue 110 Winter 2018 19 By Hamptonhalls Issuu

Course Material Chemical Pdf Mill Grinding Sodium Carbonate

Barium Chloride Dihydrate
Solved Solve This Chemistry Problem With Solution So That I Will Have A Course Hero
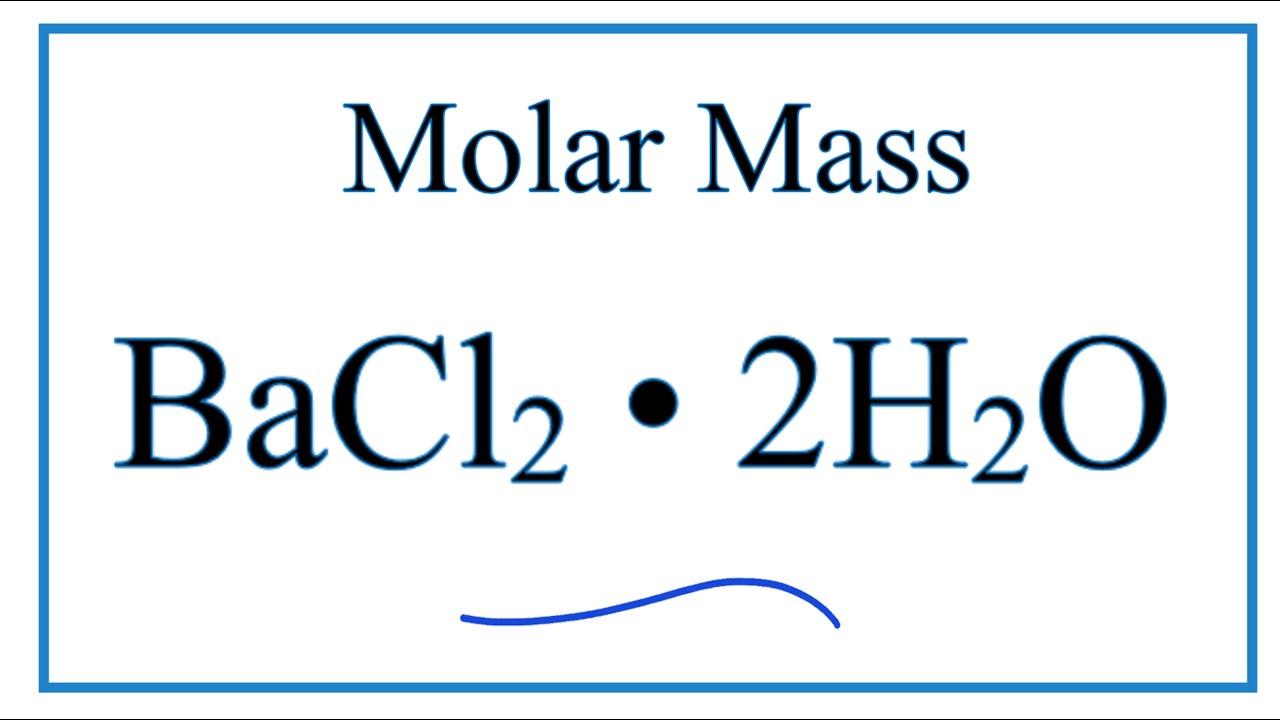
How To Find The Percent Composition By Mass For Bacl2 2h2o Youtube

Solved Use The Table Below To Answer The Following Chegg Com

How To Find The Percent Composition By Mass For Bacl2 2h2o Youtube

How To Find The Percent Composition By Mass For Bacl2 2h2o Youtube

Solved Use The Table Below To Answer The Following Chegg Com

How Many Grams Of Barium Chlorine Bacl2 Are Needed To Prepare 100 Cm 3 Of 0 250 M Bacl2 Solution